The release of V3000 representation of enhanced stereochemistry by Biovia was a great step forward, but it does introduce another level of complexity. Here, I’m pondering the usefulness of even more specificity…
From a chemist’s point of view, the good stuff is:
- I can represent a compound that I’m sure is a pure enantiomer, but not sure which one, using the OR designation.
- I can represent a mixture of enantiomers using the AND designation.
- If I have a single pure enantiomer, and I know which one it is, I can use the ABS designation.
- If I have multiple stereocenters that are connected in a relative configuration, but I’m not sure about the absolute configuration, I can create an OR group.
- If I have another stereocenter that is not connected to that relative configuration, and I know it’s a specific relative configuration, I can mark it OR.
- If I have another stereocenter that is not connected to that relative configuration, and I know it is a mixed center, I can mark it AND.
The bad stuff is:
- Wait a minute, that last example of good stuff is confusing as heck.
- How many stereoisomers are there? – There’s no tally as you add them.
- What if I got something wrong? – Can I (automatically) enumerate all the substituent structures and look at them?
- What if I have a partially purified enantiomer – like it’s 90% one enantiomer, and 10% the other.
- How do I represent that ratio?
- What if I’m sure I have a single stereoisomer, and I’m 90% sure that the stereocenter is the R configuration (but not 100% sure). How do I represent that?
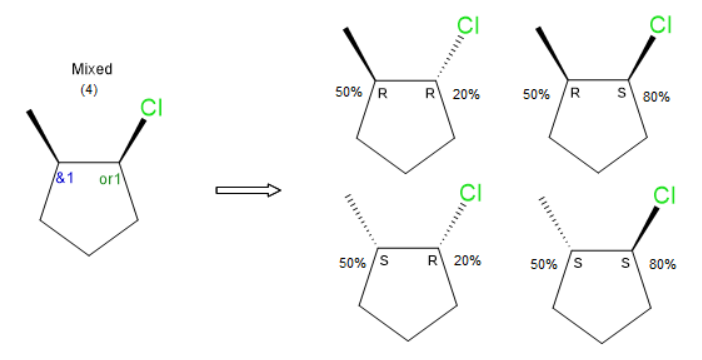
I think the central issue is the inability to enumerate and view all substituent structures. Once that is a possibility, one can envision setting percentages for individual stereoisomers. For OR atoms/atom groups, percent certainties could be set for the two structures possible structures. If there is an unrelated AND atom/atom group in that same molecule, quantity percentages could be set for the two possible structures. Since there are 2 non-ABS stereocenters in this example there are 4 stereoisomers. The ability to specify this information would be valuable in chemical registration system. Default values for both would be 50%.
Setting / displaying this additional information would require some enhancement to existing drawing tools. I’m imagining something like image below, where the percentage is input/represented in association with the stereocenter.